Oncology Xenograft Models: Using Mice To Test Cancer Treatments
Table Of Interest
Today, you and I will quickly take a look at the topic “Oncology Xenograft Models: Using Mice To Test Cancer Treatments”.
This has become necessary as we have sen overtime that several individuals have been searching for topics related to the above topic Oncology Xenograft Models: Using Mice To Test Cancer Treatments.

However, if you are among those that have been searching for answers to [mouse cancer models, mouse models for cancer research, syngeneic mouse model, preclinical cancer models, animal models in cancer research, xenograft model definition, xenograft mouse model protocol, immuno oncology mouse models, Oncology Xenograft Models: Using Mice To Test Cancer Treatments], then you can see that you are not the only one.
Nonetheless, you shall get all this information right here on this blog.
Oncology Xenograft Models: Using Mice To Test Cancer Treatments
An oncology xenograft model usually refers to a mouse that can grow human cancer cells 1.
Often these mice are immunocompromised so their immune system won’t attack the foreign cells 2.
Tumor cells are injected into these mouse models and given several weeks to grow.
Once the tumor is actively growing, scientists can test different treatments on the cancer to see what is and isn’t effective.
The strain of mouse used in this experiment can have a big impact on the results.
Originally, researchers used strains without functioning immune systems 3.
This gave the greatest chance for the tumor cells to grow, but the immunocompromised environment they grew in wasn’t very realistic compared to a human body.
The development of humanized mice provided a potential solution to this problem.
Humanized mice are genetically engineered to be born with a non-functional immune system, but they can be injected with human bone marrow to create an immune system that functions similarly to that of a human 4.
This provides a much more realistic environment for tumor cells to grow, but these mice require more work to prepare.
A variety of tumors and cancerous cells lines can be injected into the mouse. In some cases, a doctor can remove a tumor from a human, cut it into tiny pieces and implant a piece into a mouse.
This is effective in examining a specific type of cancer, but the cells may not grow depending on the type of cancer and the strain of mouse. To get around this, researchers may use established cell lines 6.
For example, if a scientist has a potential treatment for lung cancer, they can find a lung cancer cell line that’s known to grow well in a specific mouse model and use them together.
Why is it important
The main reason researchers use Xenograft models is to save time.
These experiments can be done within a few weeks while comparable experiments, like genetically engineering a strain of mice that grows a particular type of cancer, would take orders of magnitude longer by comparison.
The several week timespan is fast enough that a patient with a novel type of cancer could conceivably get a viable treatment plan before the cancer spreads too far 7.
The other advantage to xenografts is the cost.
See Other Articles People Are Reading
- Cry Me A River – From Biology To Psychology Of Tears
- Redefining ‘Health’ For The 21st Century | Health Care Changes In The 21st Century
- Missile Technology – The Weapon Of Destruction
- Discussing Day-to-Day Fear | Types Of Fears & How To Overcome Them
- Electromagnetism – Antenna Radiation Patterns | How To Read Antenna Radiation Patterns
- Do Robots Really Have Emotions? | Can Artificial Intelligence Feel Emotions?
A lab can maintain a strain of immunocompromised or humanized mice without too much trouble and grafting cancer onto groups of mice doesn’t require expensive reagents or equipment.
Xenografting also bypasses some of the need for additional analytics.
A cancerous cell’s genome may not need to be sequenced right away if a xenograft reveals a viable treatment.
Unfortunately, the low time and cost of xenograft mouse models comes at the loss of accuracy and knowledge. Just because a treatment works in this type of oncology model doesn’t mean it will work in a patient.
It also doesn’t give researchers much of a clue as to why a treatment works. They’ll still have to do rigorous genetic analysis to determine the genetic mutations that led to the cancer and even further experiments to determine what the treatment is doing to the tumor cells.
Despite these limitations, xenograft models are such an integral part of cancer research that numerous biotech companies sell humanized mice 8 and cancerous cell lines 9.
The academic field is flooded with papers that attempt to improve upon our current set of xenograft models and the ways we can use them. The pace of research on this subject doesn’t seem to be slowing down either.
To give you an idea of the type of work in this field, I’m going to do a deep dive of a recent paper that uses a xenograft model to validate a potential cancer drug.
Testing A drug That Prevents Ewing Sarcoma Metastasis
The paper “Inhibition of porcupine prolongs metastasis free survival in a mouse xenograft model of Ewing sarcoma” by Hayashi et al. 10 is a great example of why a researcher might want to use xenograft models.
Ewing sarcoma is a rare type of cancer that can usually be cured, but has to be treated aggressively because it has a tendency to spread quickly 11.
This tendency to spread, also known as metastasis, is what the authors of the paper wanted to focus on.
Previous work had identified a drug called WNT974 that inhibits a key pathway leading to metastasis known as Wnt signaling 12, and the authors wanted to see if this drug was effective on Ewin sarcoma.

I do want to take a moment and point out that WNT974 isn’t the most helpful name for this drug.
It barely gives us a clue as to what the drug does (Wnt inhibition), and it isn’t the original name of the drug (LGK974).
Add the fact that the drug targets Porcupine, not the animal but a Wnt-specific acyltransferase, and you’ve got a whole mess of confusing nomenclature. To minimize confusion, I’m only going to call the treatment the “WNT974 drug” from here on out.
The first half of this paper involves a bunch of tests that show how the WNT974 drug affects independently growing Ewing sarcoma cell lines. In brief, the researchers showed that this drug inhibits the Wnt pathway and downregulates metastasis related genes.
These results were promising, but they don’t guarantee that the drug will have the same effect when the cancer is progressing through an actual host. Thus, the authors turned to xenograft models.
The initial tests with xenograft models were fairly simple.
They injected two groups of mice with Ewing sarcoma cell lines and treated one group with the WNT974 drug while keeping the other group as a control.
They compared tumor growth and mouse survival between the two groups.
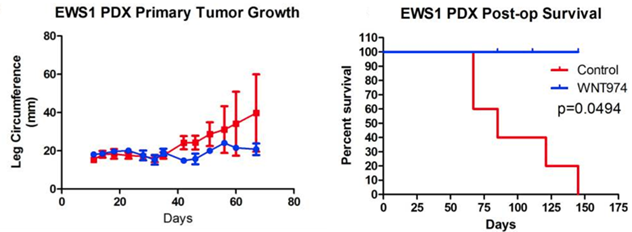
The results when using the EWS1 Ewing sarcoma cell line are shown to the right. As you can see, the drug treatment had minimal effect on the growth of the tumor, but mouse survival still dramatically improved.
All the control mice died while all the WNT974 drug treated mice survived. These results followed the authors expectations. Since the drug inhibits metastasis, it won’t get rid of the original tumor, but it will prevent the tumor from spreading.
Now that the authors showed that the drug could increase survival rates, they wanted to confirm that it specifically inhibited metastasis in vivo.
To do this, they performed a similar experiment in which mice implanted with Ewing sarcoma were treated with and without the WNT974 drug. However, instead of looking at survival rates, they looked at the lungs of all the mice.
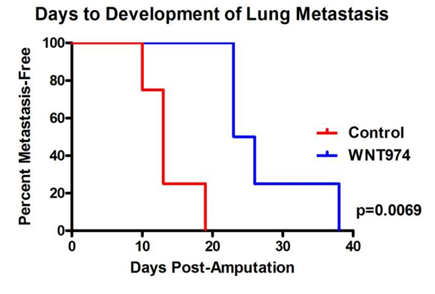
They were specifically looking for the spread of cancer to the lungs, or in other words, its metastasis.
They found that the development of lung metastasis was significantly inhibited when mice were treated with the WNT974 drug.
As a final test, the authors injected Ewing sarcoma cell lines directly into the veins of mouse tails.
They reasoned that by injecting the cancer cells into the bloodstream, they bypassed the initial steps of metastasis.
In this experiment, they found that treatment with the drug WNT974 didn’t have a significant impact on the survival rates of the mice. This suggests that the WNT974 drug inhibits the early steps of metastasis.
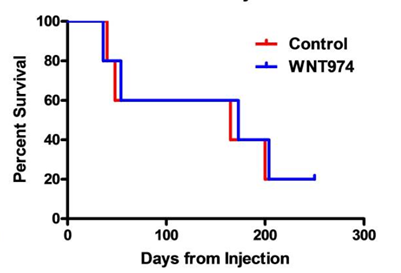
I chose this paper to review because it does a great job of showing how xenograft models can contribute to a good research project. The authors had used a number of preliminary experiments to show that the drug WNT974 was an effective treatment against Ewing sarcoma.
However, it was necessary for them to show that this drug was effective in a model that closely resembled cancer progression in a human in a reasonable amount of time.
With these criteria, xenograft models were just about the only viable option they could take. This option was nevertheless successful and gave them informative results that rounded out the paper.
The authors were also able to show that xenograft models can do more than a simple demonstration of survival rates. Their work showed that the drug specifically inhibited early stages of metastasis.
That’s the much we can take on the topic “Oncology Xenograft Models: Using Mice To Test Cancer Treatments”.
Thanks For Reading
See Other Articles People Are Reading
- Cry Me A River – From Biology To Psychology Of Tears
- Redefining ‘Health’ For The 21st Century | Health Care Changes In The 21st Century
- Missile Technology – The Weapon Of Destruction
- Discussing Day-to-Day Fear | Types Of Fears & How To Overcome Them
- Electromagnetism – Antenna Radiation Patterns | How To Read Antenna Radiation Patterns
- Do Robots Really Have Emotions? | Can Artificial Intelligence Feel Emotions?
Leave a Reply